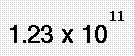What is the geometry of the methane molecule?
The simplest hydrocarbon , methane is a gas with a chemical formula of CH4 and a molecular weight of 16.04.
-------------->spin on -------->-
spin off
------>space fill/cpk
-------->stick
----> ball-and-stick
To Rotate the Molecule--->Left Click and Drag
To Zoom-->>Left Click + hold Shift button and Drag Vertically
Jsmol Menu --->>Right-Click
Try this!!
Change to wireframe first:
Click on right mouse button over box
Style --> Schemes --> wireframe
Label atoms:
Style -->Label ---> atom number
To measure angles: hold left mouse bottom over atom and double click on atom 1, drag to second atom (center atom) single click center atom, drag and double click atom 3.
Measure the following angles:
<415, <512, <213, <314
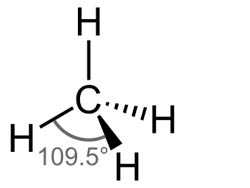
Below is shown the molecule Methane.
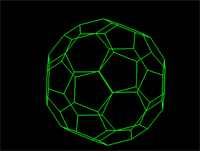
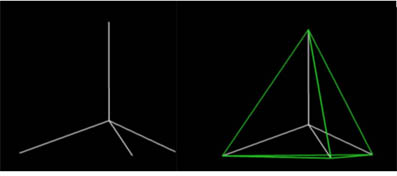
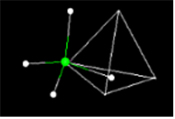
What polyhedral stucture would you think the methane molecule best represents? View the flash video below to see...

About Methane:
Methane is a principal component of natural gas. When a single molecule of methane is burned in the presence of oxygen releases one molecule of CO2[carbon dioxide) and two molecules of H2O (water).
CH4 + 2O2 ---> CO2 + 2H2O
The strength of the carbon hydrogen covalent bond in methane is among the strongest in all hydrocarbons.
Pure methane is odorless, but when used as a fuel it is usually mixed with small quantities of strongly-smelling sulfur compounds such as ethyl mercaptan to enable the detection of leaks.
Methane is a greenhouse gas with a global warming potential of 22 (meaning that it has 22 times the warming ability of carbon dioxide). Methane results from the decomposition of certain organic matters in the absence of oxygen. It is therefore also classified as a biogas.
Assessment -- Try these questions--
1) What is the measurement for any of the H-C-H angle to the closest tenth degree?
Please enter your answer in the space provided:
2) What is the measure of the C-H bond in methane?
3)How many faces does a tetrahedron have?
MATHMOL
- Activity 1: Measuring length and distance at the molecular level
- Activity 2: Geometry-of-1-Dimension
- Activity 3: Geometry of 2- Dimensions
- Activity 4: Geometry of 3-Dimensions
- Activity 5: Introduction to Molecular Modeling using Jsmol
- Activity 6: The Geometry of Crystals
- Activity 7: Summary Sheet by Students
- Activity 8: What is the Geometry of the Methane Molecule
- Activity 9: Geometry of the Crystal Structure of Ice
- Activity10:Geometry of the Benzene Molecule