Introduction to Molecular Modeling for K-12 students- A STEM Activity
The following activity uses Jsmol instead of the previous Jmol applet. Jsmol utilizes Javascript and can be used ontablets and runs well in the Mac OS. It is fatest if used with the Chrome browser.
Note: Next Generation Science Standards (NGSS) -- Struture and Properties of Matter - Emphasis is on developing models of molecules that vary in complexity..."Examples of molecular-level models could include drawings, 3D ball and stick structures, or computer representations showing different molecules with different types of atoms.
PART Ia:
Chemical formulas are a shorthand way of representing chemical substances. The chemical formula for water is another way of saying that each molecule of water contains two hydrogen atoms and one oxygen atom. In the molecular model shown above each atom (element) is represented by a sphere of different color. Try answering each of the following questions on a separate sheet of paper. Some of the questions allow you to check your answer online. Be sure to click the submit button after you type your answer.
Question 1: In the model of water shown in the box above, what color is the oxygen molecule?
Question 2: What color is the hydrogen molecule?
Question 3: Why do you think it is important to view images in 3-D? __________________________
Without moving the image on the left can you tell if these two molecules are the exactly the same?
From what you can see it looks like the image of the molecule on the left has only 4 atoms (1 carbon and 3 hydrogens) while the molecule on the right has 5 atoms (1 carbon and 4 hydrogens). By viewing molecules in 3-Dimensions it is possible to getter a more accurate perspective of the molecules structure and geometry. Molecular modeling gives us this ability.
Using molecular modeling it is possible to rotate, zoom in and out, and translate molecules in the X-Y axis.
Try these activities with your partner.
Take the mouse cursor and put it over the molecule (above) on the left. Hold the left mouse button down, and slowly move the mouse a little to the left, then a little to the right. Now move the mouse slowly up and down. You can now see that the two molecules are the same.
The molecules are both methane (CH4).
If you want to get a closer look try this. Hold the shift button down and the left mouse key down at the same time. Put the mouse cursor arrow inside the box and move the mouse up and down. The molecule should change size.
________________________________________
Try each of the following commands while moving the mouse cursor over the molecule shown below. The molecule you are moving is aspirin.
CONTROL
PANEL
(put mouse cursor in box then:)
|
Question 4: How many different atom types (elements) does aspirin have?
Try this: Click right mouse button over image --->Style --> Labels --> with element symbols
Above is shown the ethanol molecule.
There are four waysthat the ethanol molecules can be displayed when using molecular
models
..
Molecular models fall into four basic categories: skeletal or line; stick, ball-and-stick, and space-fillied or CPK.
To change from one model to the another
Try this now : hold the right mouse key down (over the ethanol molecule) ---> Style--->Scheme --->Choose your model (wireframe, stick, ball and stick, or CPK (space filled)
Try changing the above structures to each of the display forms. With your partner identify the different molecular model types for ethanol.
Each model has its advantages and disadvantages when used for display purposes. Discuss with your partner what may be the advantanges and disadvantages in using each model. Below are images of the four models.
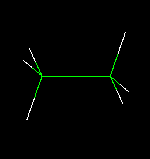
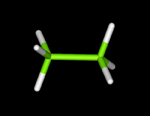
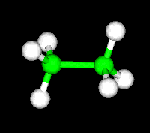
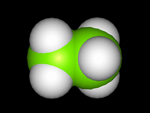
| Wireframe
Model | Stick
Model | Ball
and Stick Model | Space
Filled Model |
Question 5: What would you expect the chemical formula of ethanol to be?__________________
You will now use molecular modeling to help you in identifying several hydrocarbons in the Alkane series.
| The table shown here gives the chemical formulas for several simple hydrocarbons (compounds containing only carbon and hydrogen). Study the pattern shown in the table. Do you recognize the pattern? Question 6: How many hydrogens would the molecule Decane (10 Carbons) would have how? |
Can you match the structural formulas above with the 3-D molecular models below? Try rotating and/or zooming in on each molecule to help identify the structure. Choose from the following: methane, ethane, propane, butane, pentane, hexane and octane.
MATHMOL
- Activity 1: Measuring length and distance at the molecular level
- Activity 2: Geometry-of-1-Dimension
- Activity 3: Geometry of 2- Dimensions
- Activity 4: Geometry of 3-Dimensions
- Activity 5: Introduction to Molecular Modeling using Jsmol
- Activity 6: The Geometry of Crystals
- Activity 7: Summary Sheet by Students
- Activity 8: What is the Geometry of the Methane Molecule
- Activity 9: Geometry of the Crystal Structure of Ice
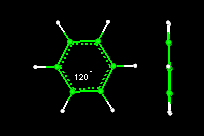
- Activity10:Geometry of the Benzene Molecule